SYLLABUS STATEMENT 1.7 AND 1.8
Last post I was talking about elements, compounds and mixtures and how there is a whole big chapter on separating mixtures. Well here’s the post on separating mixtures. There are a bunch of different mixtures you could have so I’ll separate it up for you.
If you have a solid and a solid:
- Is one solid magnetic? It can be removed by a magnet
- Does one dissolve in water? Add water, remove the insoluble solid and evaporate the water to get the soluble solid.
- Does one float and one sink? You can scoop the solid that floats off the top and filter the solid that sinks.
If you have a liquid and a liquid:
- Does one have a lower boiling point than the other? You can use distillation to remove the more volatile liquid.
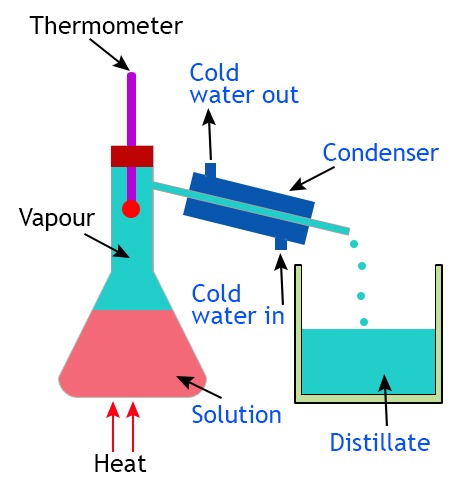
This is distillation equipment. You first heat the mixture of liquids. The more volatile liquid evaporates. The vapour goes into the condenser where is is condensed back into a liquid which lows out the other side into a beaker. Cold water is pumped through the condenser to make sure the condenser is kept cool.
If you have a solid and a liquid
If there is ever a solid and liquid mixture then you only need to filter it.
WHAT IF YOU HAVE MULTIPLE LIQUIDS MIXED?
WHAT IS CHROMATOGRAPHY?
Chromatography separates the different components of a mixture of liquids. It works because different components of the mixture are more soluble than others.
HOW TO DO CHROMATOGRAPHY
Chromatography is actually really easy, you can even do it at home! All you need is some filter paper, a beaker of water something to clamp the paper with and a few pens.
First things first, get your filter paper and draw a few spots of ink in a straight line near the bottom of the paper. Leave about a 1cm gap between the bottom and your ink. Then get your beaker and fill the bottom with a little bit of water. Clamp your paper so that the bottom is in the water, but the water does not touch the ink. Then just leave it for a few days. when you come back your ink should have risen up the paper like this.

As you can see the dye has split up into different components as the water rises up the paper. They separate because the water holds onto some dyes stronger than others (NOTE: Simple explanation, there is a lot more to it than that)