The 2019 Nobel Prize in Chemistry was awarded three chemists for their role in the chemistry behind making powerful and safe lithium ion batteries; John B. Goodenough, M. Stanley Whittingham and Akira Yoshino.
Their discoveries
Goodenough, Whittingham and Yoshino, unlike previous joint prize winners, worked separately and at different times working on each others previous discoveries to create the lithium ion batteries we use today.
Their advances on the lithium battery has had a huge impact on the digital world we live in today, leading the way to powerful, long lasting and safe lithium batteries for use in phones, laptops, electric vehicles and so much more.
Lead-acid batteries - the predecessor
Before the lithium battery were lead acid batteries, used to start cars and power other devices. Lead acid batteries are great for starting cars and trucks because they are inexpensive but these batteries are extremely heavy. Why? because lead is one of the densest elements in the periodic table.
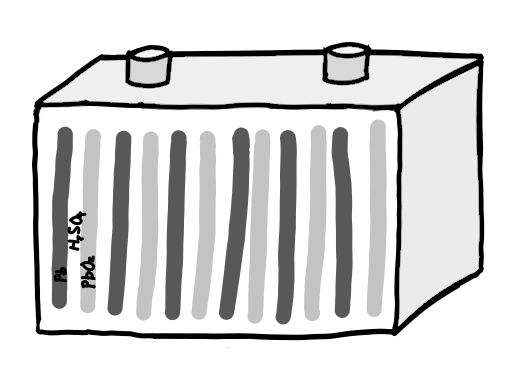
They are also high maintenance and only have a lifespan of about 300 charges compared to the modern lithium battery’s lifespan of about 5,000 charges.
The difference with lithium ion batteries
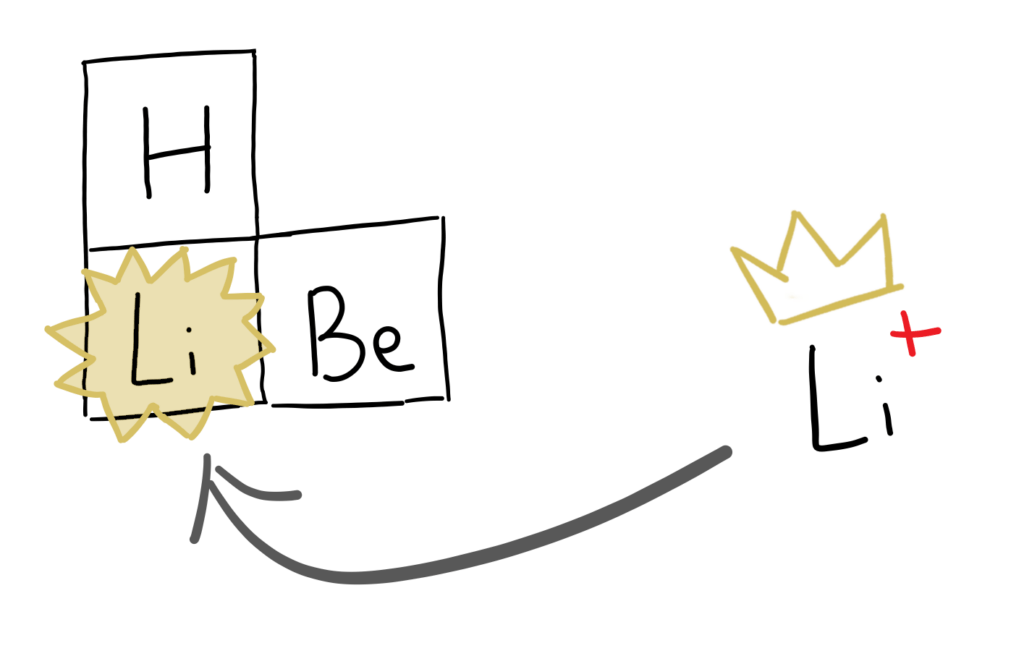
Lithium, being the third element in the periodic table, is incredibly light, giving it great potential to make lightweight powerful batteries. These batteries also have a huge energy density, allowing one small battery to hold a relatively large voltage.
Not only this, the modern lithium batteries can hold enormous amounts of energy and after many charging cycles, they can still maintain a majority of their original capacity.
Taming the beast
Lithium, the first alkali metal, is highly reactive and has to be stored under oil to prevent it from reacting with air. Just like it’s brothers and sisters in the alkali metal group of the periodic table, it reacts quite violently with water. This meant that it was not only challenging to build the battery, but also to make it safe. The first generations of lithium ion batteries caused many fires that have to be extinguished using expensive chemicals (remember? Lithium reacts violently with water).
This was one of Yoshino’s key breakthroughs – building a lithium ion battery that contained no pure lithium, making it viable for consumer use.
What's next for battery technology?
As we move towards a future filled with more battery powered electrical appliances, the next generation of batteries are being developed. Lithium is an especially rare element on earth, making production costs high. Lithium’s sister element, potassium, is much more common and is being used to develop more powerful potassium ion batteries that can hold more charge.
Of course, potassium being an alkali metal like lithium means it holds many similar challenges regarding it’s stability and reactivity. If you thought lithium was reactive, you should see potassium.
Why is this Nobel Prize is especially significant?
The invention of the lithium ion battery has had a huge impact on the lives we lead today. The device I’m using to type this and the one you’re reading it on are likely both battery powered, our clocks, torches, calculators and watches are battery powered and soon hopefully most of the vehicles we use to travel around will be too.
This year’s Nobel Prize winning discovery in chemistry truly impacts everyone. Their discovery paved the way for current and future developments in moving towards technological advancements and a more environmentally friendly future.
The story and details of their discoveries