WHAT THE HELL IS EQUILIBRIUM?
EQUILIBRIUM SHIFTS
The position of the equilibrium is related to the amounts of substances on each side of the equilibrium reaction. For example :
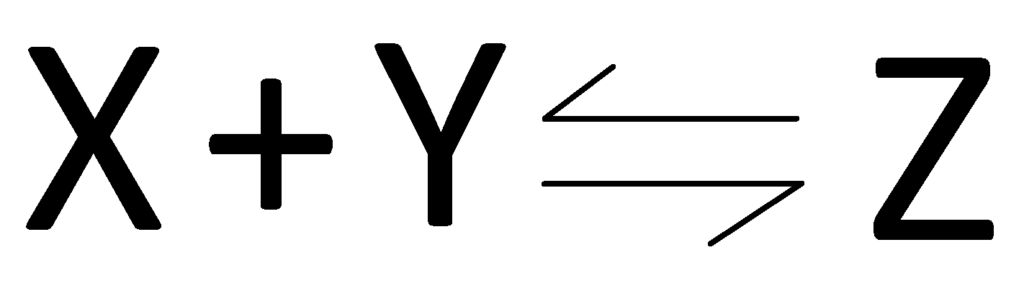
If there are much higher concentrations of X and Y than Z, then the equilibrium lies to the left. If there is a higher concentration of Z than X and Y then the equilibrium lies to the right.
LE CHATELIER'S PRINCIPLE
Le Chatelier’s Principle basically tells you which way the position of the equilibrium will move if you make changes to the equilibrium – like changing the concentrations of some of the reactants / products or change the temperature or pressure. Le chatelier’s principle says that the equilibrium will shift to oppose the change you made.
CHANGING CONCENTRATIONS
Lets say in the equilibrium above, I removed some of substance X from the equilibrium. The equilibrium would shift left to oppose the change by making more X (and Y) from Z. The same works the other way, if I removed some of substance Z, more X and Y would react to form Z to make up for the amount you just removed.
CHANGING TEMPERATURE
To know which way the equilibrium shifts when you change the temperature, you need to know the ΔH for the reaction. For Example :
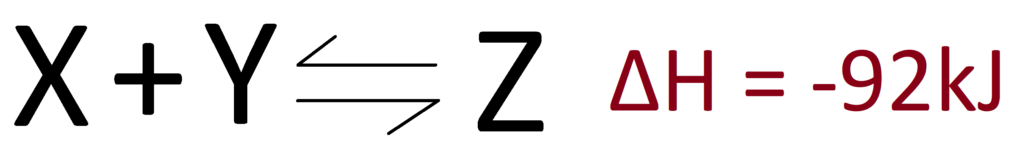
In this example, the forward reaction is exothermic (because ΔH is negative). Le Chatelier’s Principle says that the equilibrium will shift to oppose the change you’ve made. So if we increase the temperature, the equilibrium will shift to decrease it. To do that, it needs to use up energy. Energy is absorbed in endothermic reactions, therefore increasing the temperature favours the endothermic reaction. This of course works the other way too! If you decrease the temperature, the exothermic reaction is favoured, because it will release heat energy and change the temperature back to what it was before.
CHANGING THE PRESSURE
A quick refresher on pressure : The more particles in a fixed volume, the higher the pressure right? Le Chatelier’s Principle shifts the position of the equilibrium to oppose the change you’ve made. If you’ve increased the pressure, Le Chatelier’s Principle needs to reduce the number of molecules, so the equilibrium shifts towards the side with less moles of gas. In our example of X+Y⇄Z, the product side has less moles so increasing the pressure will shift the equilibrium right.
I HOPE THIS WAS USEFUL!
So sorry I’ve been so inactive – I’ve had a hell of a lot to do!