SYLLABUS STATEMENTS 1.4 - 1.6, 1.9 - 1.10, 1.13 - 1.15
HOW DO WE KNOW ATOMS EVEN EXIST?
Well, there are a few different ways actually, but I’ll stick to the ones you need to know for Chemistry. The first one is diffusion. Scientists back in the day observed people smoking and thought why does the smoke disappear? Then they thought well for it to move there must be something pushing it (Newtons 2nd Law!) and the only reasonable culprit was atoms. This was backed up when a Mr. Brown was looking at pollen grains in water under a microscope when he realised that the pollen grains were jiggling around. He called this motion Brownian motion after himself like they all do.
Then later on the genius Albert Einstein came along and made the link between Brown’s jiggling pollen grains, diffusion and atoms and there it was. Since then our idea of what atoms are has changed, from the plum pudding model, to the nuclear model and finally to the current quantum mechanical model of the atom. For GCSE forget about the quantum mechanical atom and focus on the nuclear model. This is the ‘right’ one.
THE NUCLEAR MODEL OF THE ATOM
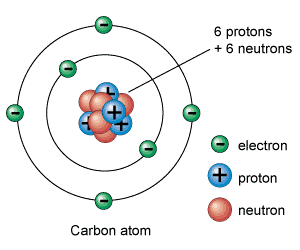
This model of the atom should hopefully be familiar to you. A nucleus of positively charged protons and neutrally charged neutrons surrounded by orbiting shells of negatively charged electrons.
MOLECULES - WHAT ARE THEY?
Molecules are basically what you get when 2 or more elements (types of atoms) chemically bond together. This is where people go wrong – a compound’s elements are chemically bonded together not just mixed, like gluing different marbles together as opposed to mixing the all together in a bowl. Once they’re glued they’re hard to separate again. There are hundreds of thousands of compounds around, you and your room are made of a lot of different compounds (this is what I find so fascinating!).
HOW ARE COMPOUNDS MADE?
You know how atoms have shells of electrons? Well as you get more and more shells the number of electrons you can fit in them increases (which is why the periodic table gets wider as you go down). Well atoms want to have full shells and they will stop at nothing to gain or lose electrons to achieve this. You can see how many electrons different elements have in their outer shells by looking at the periodic table – invented by the one and only Dmitri Mendeleev!
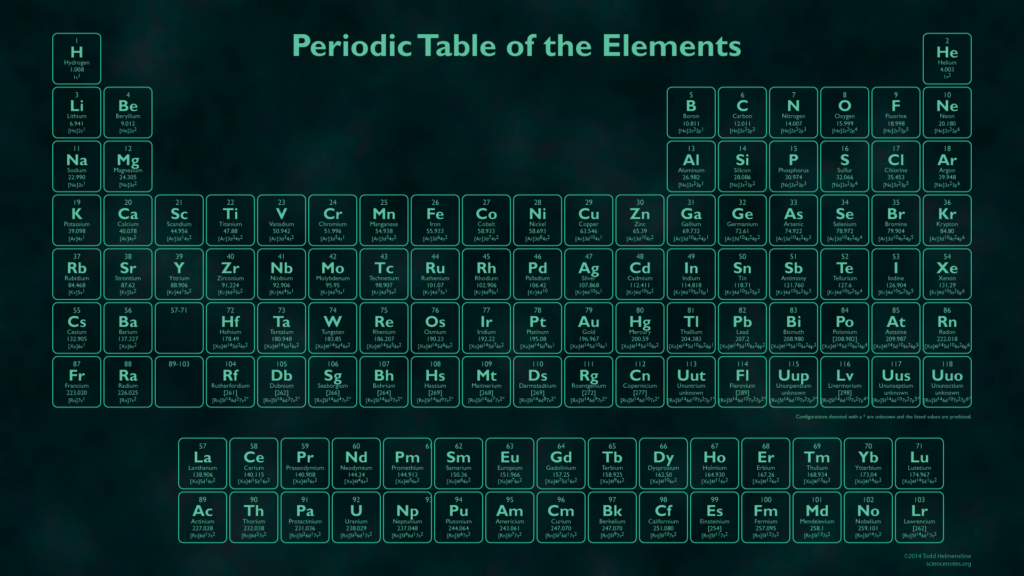
Hopefully you can see that all the elements in group 1 (the left most column) have 1 electron in their outer shell while the elements in what you might call group 8 have full shells (with 8 electrons depending on how many shells you have).
An element with 6 electrons in it’s outer shell that only needs 2 more electrons to make a full shell like Oxygen will try and bond with any element that can help it complete its outer shell.
The ways in which atoms can do this is something to discuss another time but basically it happens.
Back to oxygen. He (or she if you want to be pc) needs 2 more electrons to complete his outer shell. One way he could do this that is very useful to us humans is to bond with 2 hydrogen atoms (each lending 1 electron each) to create H2O (it won’t let me subscript the 2 ok grammar nazis) which is a particularly important molecule unless you want to die delirious and with bad leg cramps, so do stay hydrated please.
WHAT THE HELL ARE MIXTURES THEN?
Mixtures however are something completely different. Luckily for you if you struggle with chemistry, they are a hell of a lot easier to understand then compounds. They are when you mix 2 substances and they don’t chemically bond at all. This like when you mix sugar into a hot cup of tea, it doesn’t react and start fizzing, the sugar just mixes with the tea which means you can remove it.
This whole thing of separating mixtures creates a whole chapter in the chemistry textbook which is good because it is relatively easy.
You may ask, if the sugar just mixed into my tea doesn’t react but only mixes, then why does it disappear? Well it dissolves. You’ve probably heard that before but it dissolves into the water because it is soluble. This basically means it has a tendency to slip away into the water and chill out in there for a bit because it is a friend of the water (NOTE: Very basic explanation. There is a lot more to it then that!). There are a load of different ways to separate mixtures but that in itself, seeing as there is a whole chapter devoted to it, requires a separate post.
THAT'S IT FOR NOW!
Let me know if this was useful or not, and what topics you want me to do down in the comments 😝.