10.2 - SL Organic Reaction Mechanisms
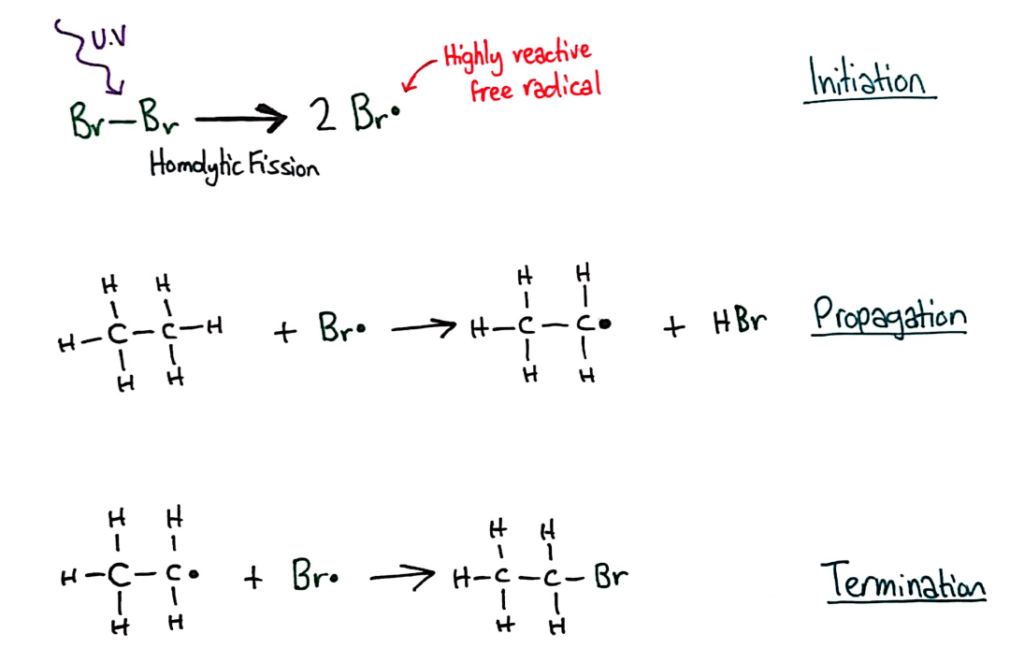
Alkanes have low reactivity and undergo free radical substitution reactions.
Alkanes are so unreactive, one of the only reactions they do is free radical substitution, where the hydrogens are replaced/substituted by highly reactive bromine radicals that are formed when bromine molecules undergo homolytic fission in the presence of UV light.
Alkenes are more reactive than alkanes and undergo addition reactions. Bromine water can be used to distinguish between alkenes and alkanes.
Addition reactions always take place over/across the double bond in a molecule. In alkenes, there are 4 main reactions that occur across the double bond, some of which are explored more deeply in Topic 20 (HL)
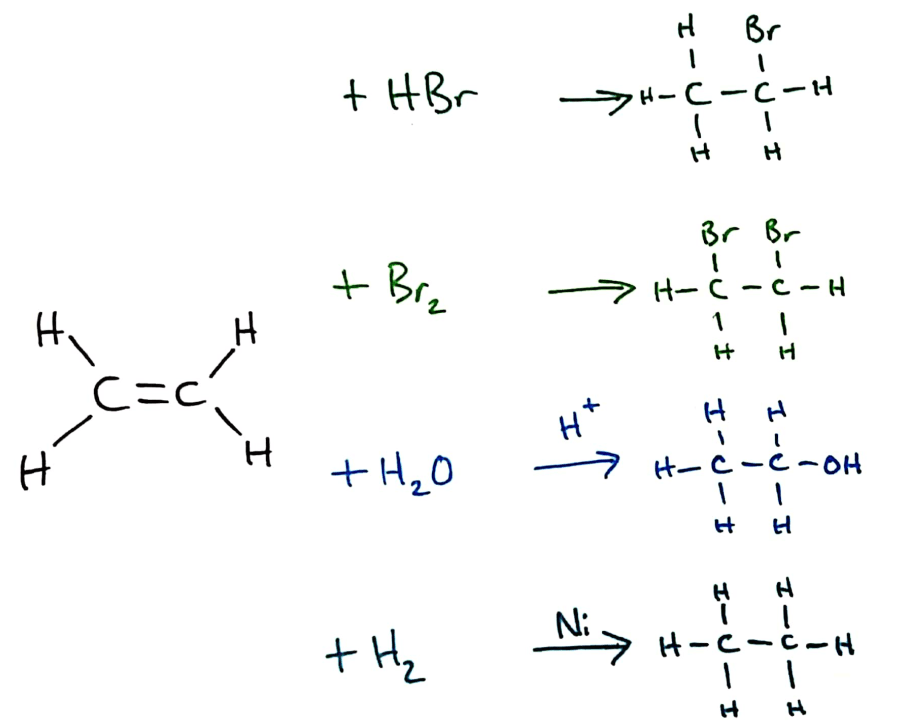
(From the top) Halogenation by hydrogen halides and halogens, hydration and hydrogenation
Bromine water can be used to distinguish between alkanes and alkenes because of the addition reaction that occurs between alkenes and bromine. If a sample of an alkene is added to bromine water, the bromine reacts and the bromine water decolourises. Alkanes will not react in the same way, and will not decolourise bromine water.
Halogenoalkanes are more reactive than alkanes. They can undergo (nucleophilic) substitution reactions. A nucleophile is an electron-rich species containing a lone pair that it donates to an electron-deficient carbon.
This syllabus statement is huge, let’s split it up a bit…
Why are halogenoalkanes more reactive than alkanes?
Alkanes
- No polar bonds or dipoles
- Non polar molecule
- Less IMFs (only LDF)
- Therefore less reactive
Halogenoalkanes
- Bond dipole/polar bond
- Can sometimes be polar molecules
- More IMFs (LDF and dip-dip)
- Therefore more reactive
Nucleophiles
Are positive seeking species that are often negatively charged and/or have lone pairs of electrons
Examples:
- OH-
- CN-
- NH3
Nucleophilic Substitution of halogenoalkanes (SL)
(The HL part of nucleophilic substitution is more complex and detailed, see topic 20 for that)
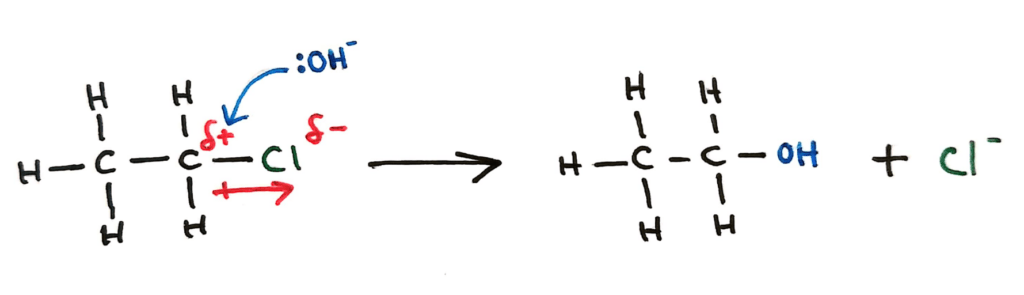
A halogenoalkane can undergo nucleophilic substitution by the hydroxide ion (strong nucleophile) to form an alcohol. The OH- ion’s lone pair is attracted to the slightly positive carbon, and manages to displace and replace the Cl. In HL we go into more detail of this reaction (SN1 and SN2).
Esterification (SL)
Alcohols undergo nucleophilic substitution reactions with acids (also called esterification or condensation) and some undergo oxidation reactions.
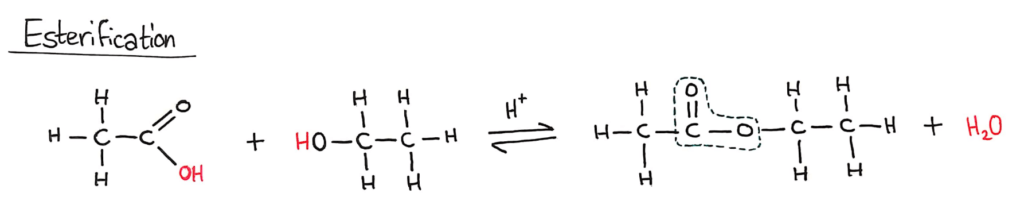
The circled part of the ester is called an ester linkage
A carboxylic acid and an alcohol will react together in an equilibrium reaction to form an ester and water. This reaction is a condensation reaction (it forms H2O) and requires a strong acid catalyst.
Oxidation of alcohols
Alcohols can be oxidised to aldehydes, ketones and carboxylic acids in the presence of oxidising agents such as acidified potassium dichromate (Cr2O7-).
Primary alcohols
Primary alcohols can be oxidised to aldehydes and carboxylic acids depending on the degree of oxidation.
Aldehydes can be produced by heating a mixture of the alcohol and the acidified oxidising agent and using distillation to collect the aldehyde (the aldehyde has a lower boiling point than the alcohol and carboxylic acid.
Carboxylic acids and ketones (different reactants/alcohols) can be produced by heating the alcohol under reflux in an excess of oxidising agent in order to fully oxidise the alcohol.
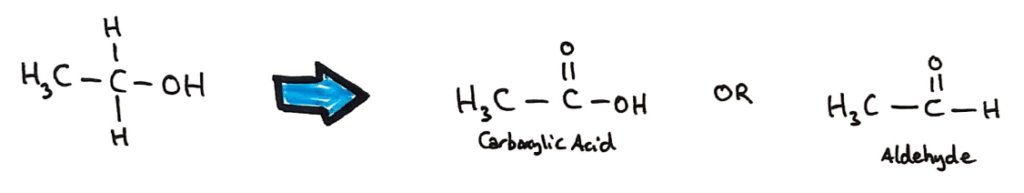
Secondary alcohols
Secondary alcohols oxidise to form ketones in the same way primary alcohols oxidise to form aldehydes and carboxylic acids.
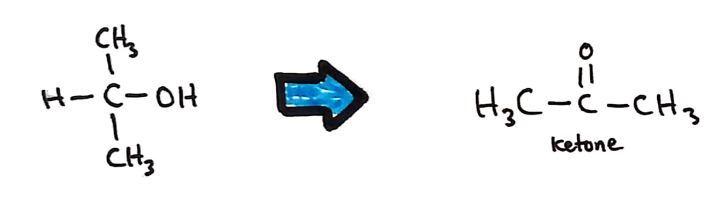
Tertiary alcohols don't oxidise 😥😥😥

Addition polymerisation
Addition polymers consist of a wide range of monomers and form the basis of the plastics industry.
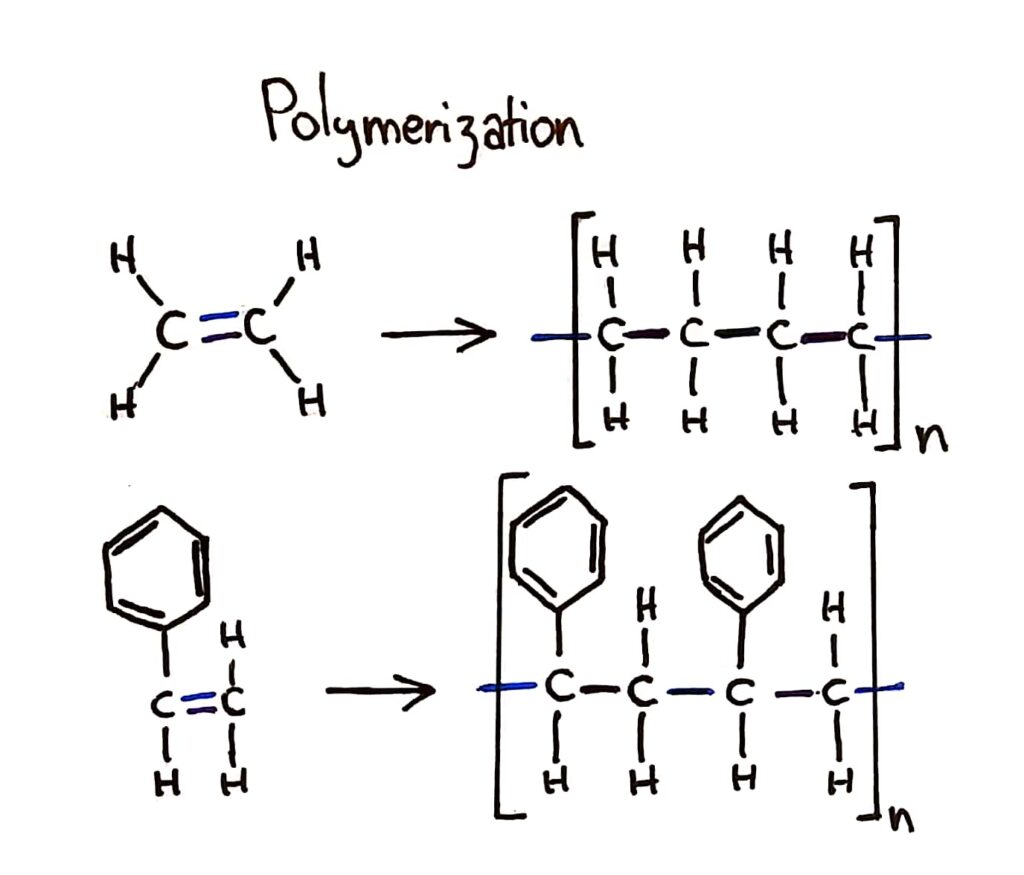
The bracket should only show 1 monomer in it but I drew 2 to show the bonding between the monomers
Addition polymers are great materials because they’re flexible, waterproof and can have a range of different properties depending on which monomer you use.
During addition polymerisation, the double bond of the monomer breaks open and forms bonds with other monomers to form a long polymer chain
Nitration of Benzene (Electrophilic Substitution)
Benzene does not readily undergo addition reactions but does undergo electrophilic substitution reactions.
Electrophiles
Electrophiles are species that are attracted to electrons and negative charges.
Electrophilic Substitution
Strong electrophiles can react with benzene and replace hydrogens because benzene has a ring of delocalised electrons, making the benzene ring very electron dense. This makes benzene very attractive to electrophiles, which are attracted to electrons.
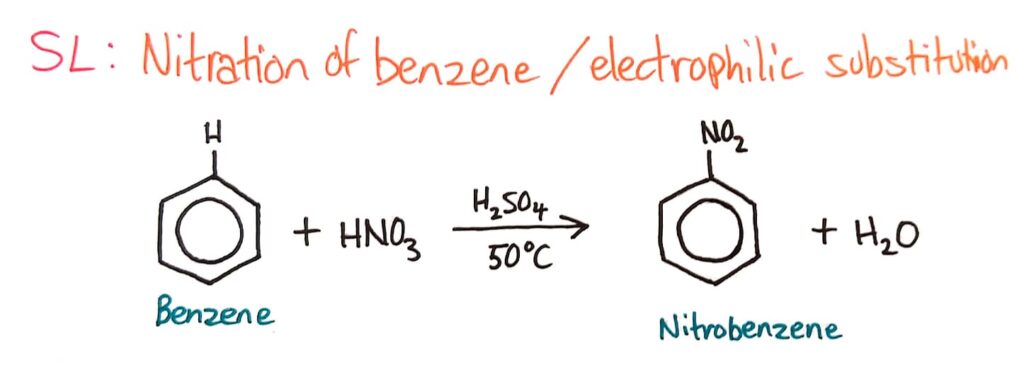
The nitration of benzene is covered in much more detail in topic 20, where you look at the involvement of the catalyst and the reaction mechanism which is awesome 
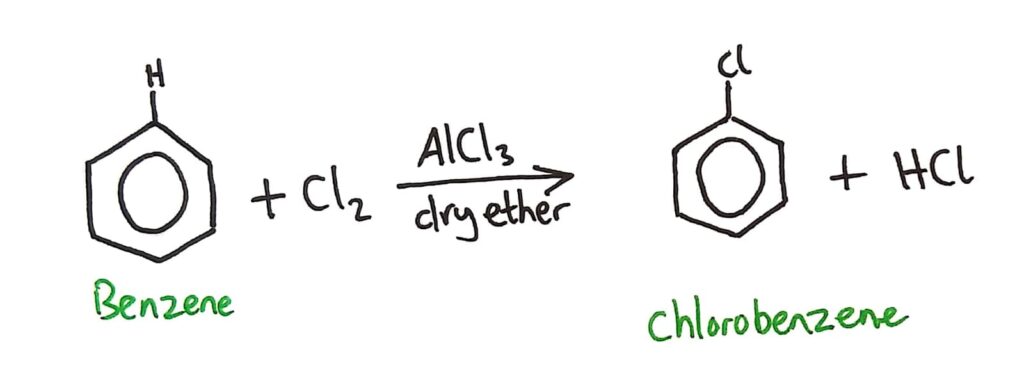
Benzene can also be chlorinated by a similar reaction that reacts benzene with Cl2 (with AlCl3 catalyst in dry ether).


thank you!!!