WHAT ON EARTH IS ELECTROLYSIS?
Electrolysis is breaking down an ionic substance using electricity. This may sound a bit confusing so let me explain it all.
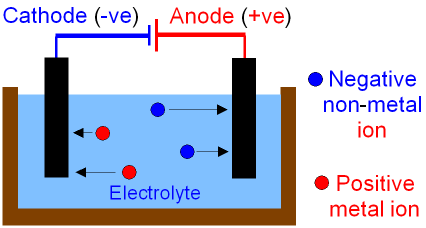
First things first, here’s a picture so you can visualize what is going on. Splitting of the compound is done by the electrodes. There are 2 electrodes, positive and negative and they have names.
WHAT ARE THE NAMES AND HOW DO I REMEMBER THEM 😰😰😰
If you remember negative ions are called anions and positive ions are called cations then this part should be relatively easy.
In electrolysis you have a positive and negative electrode right? Well which ions will be attracted to the negative electrode? The positive ions or the cations right because positive and negative attract. So the negative electrode is called the cathode because cations are attracted to it.
It goes the other way too, the positive electrode will attract the negative anions and is therefore called the anode.
WHAT SORT OF ELEMENTS FORM AT THE ELECTRODES?
Have a look at the nearest periodic table (likely located several kilometres away). Which sorts of elements will be attracted by the cathode or will form cations? Elements in groups one and two of the periodic table are most likely to lose electrons and become cations so the cathode usually is where the metals form and the anode is usually where non metals form.
HOW DO YOU DO SEPERATE STUFF USING ELECTROLYSIS?
So lets say you have an ionic compound, salt (NaCl) for example. But you don’t want salt you want it’s components, you want the sodium and chlorine it’s made of. Easy way to do this, electrolysis.
Electrolysis works by putting the electrodes into a molten or dissolved ionic substance (forget about dissolved for the moment) which connects the circuit by attracting the ions to the electrodes where they gain or deposit electrons.
BUT IF I STICK ELECTRODES IN TABLE SALT NOTHING HAPPENS...
Why does this connect the circuit I hear you ask? If I stick electrodes connected to a battery in some salt it doesn’t work. Well this is why you need to melt or dissolve the substance. Melting the substance means the ions in the substance are free to move around and are attracted to the electrodes, same if it’s dissolved. In a solid the ions are all stuck in the strong ionic lattice and they cannot break free to go visit the electrodes.
HALF EQUATIONS
Great. Equations. Well actually these are a lot more simple than the other chemical equations you will come across. Why half equations? Why not whole equations? Well they’re half equations because they only describe half of an electrolysis experiment, either the anode or cathode.
As an example lets go for an electrolysis of lead bromide (PbBr2)
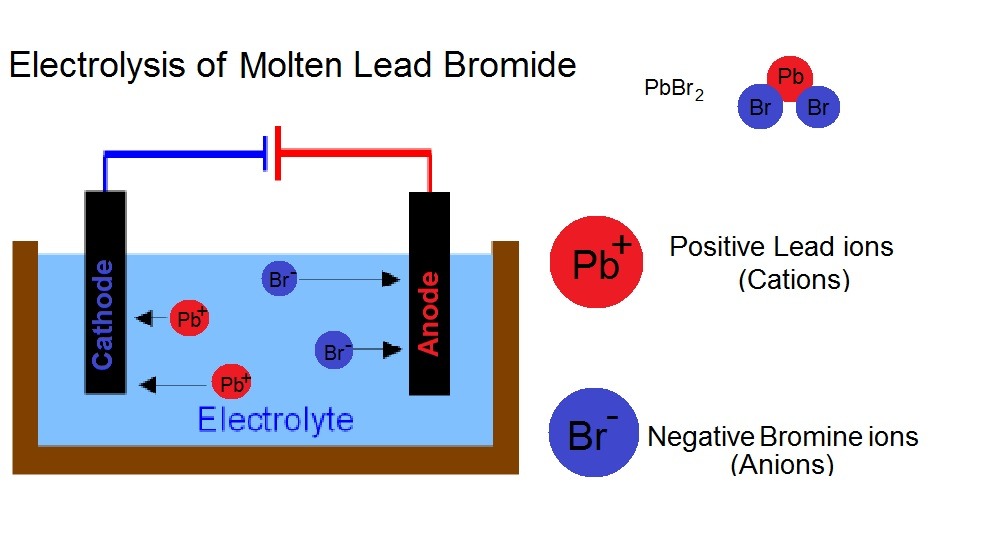
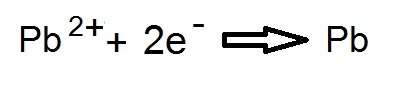
WHAT ABOUT THE ANODE?
For the other half of the electrolysis (the anode side) we also need to write a half equation. If you remember, the anode will attract the anion (the negative ion) which in this case is the bromine.
The bromine ion is Br – (minus) meaning it has an extra electron. So to make Bromine atoms we need to to take away this extra electron. The GCSE way to do it through is instead of taking away the electrons on the left side, we add them on the right side. So instead of being Bromide (bromine ions) minus electrons —> Bromine, we write Bromide —> Bromine + electrons
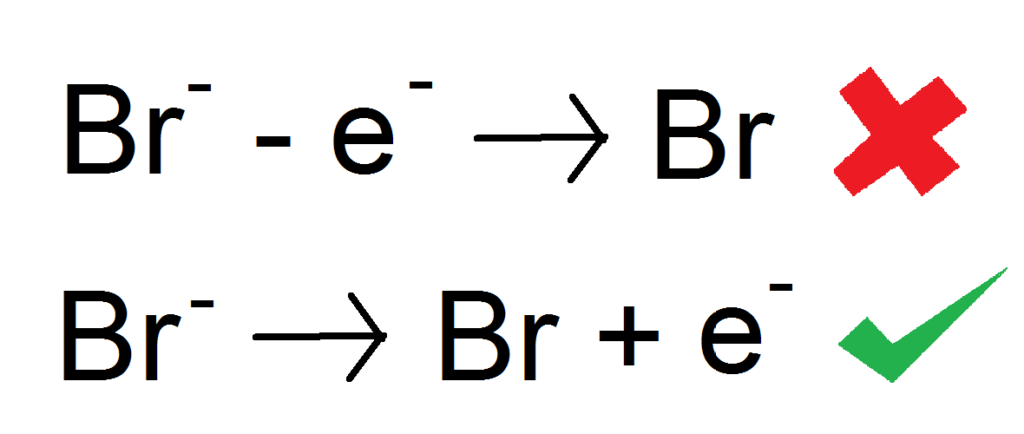
NOTE: THIS IS NOT A CORRECT HALF EQUATION. READ ON TO FIND OUT WHY.
There is one thing we have forgotten with the above half equation. Balancing! It looks pretty balanced right? Well guess what, it isn’t. We have forgotten that halogens are diatomic and join up to form Br2. We need to account for that in our half equation. This is honestly so easy, we literally just multiply everything by 2
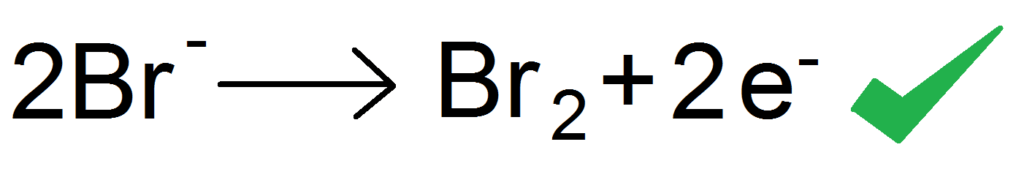
RIGHT. ON TO SOLUTIONS.
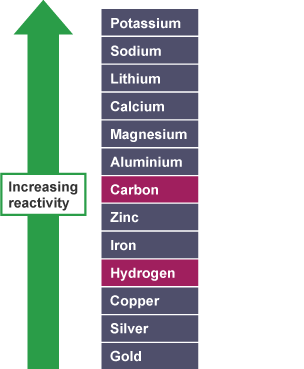
For the Cathode look at the reactivity series. Basically whichever element is lower down the reactivity series or is less reactive will go to the cathode. So in an electrolysis of Sodium Chloride you will get Hydrogen gas forming at the cathode because hydrogen is lower down the reactivity series (less reactive) than Sodium.
For the anode it’s a similar story. Basically at the anode you will either get a halogen or oxygen. So if you have something like copper sulphate or copper carbonate you will get oxygen at your anode and if you have something like Copper Chloride at your anode you will get Chlorine
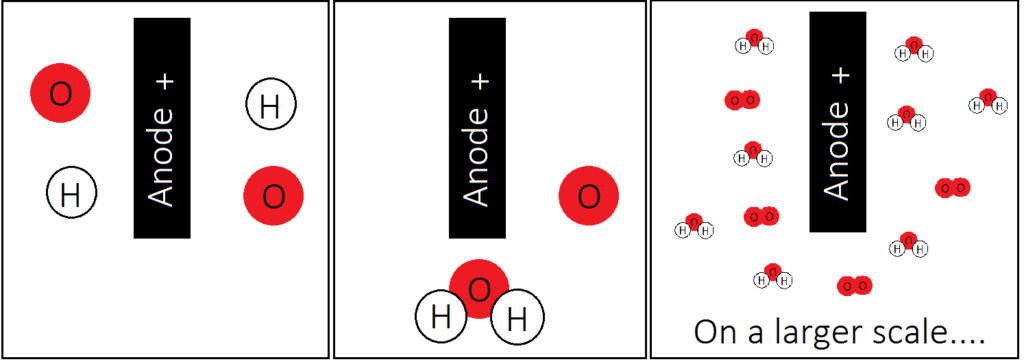
WHY DO WE GET OXYGEN?
We get the oxygen from the OH- ion. The OH- ion discharges (get’s rid of it’s extra electron) at the anode. So now we have oxygen atoms and hydrogen atoms at the anode in a ratio of 1:1. hydrogen and oxygen react to form water, but water requires 2 hydrogens, not 1. So when the oxygen and hydrogen react we end up with extra oxygen
HALF EQUATIONS
They’re almost exactly the same as the ones I showed you before. For metals and halogens they’re exactly the same as they would be molten. The equations for hydrogen at the cathode and oxygen at the anode are a tiny tiny tiny bit different.
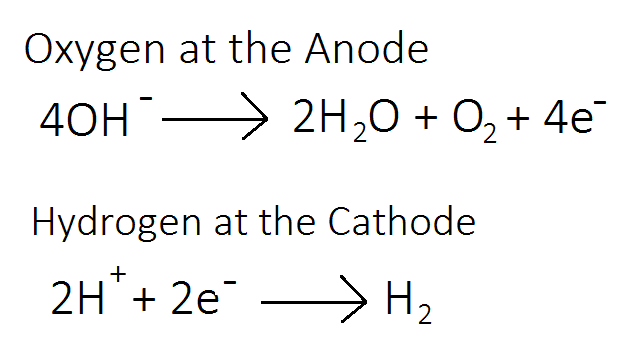
AND THAT'S ALL YOU NEED TO KNOW ABOUT ELECTROLYSIS!
Once again I hope this has been useful to you guys and if there is something you want me to explain, write it down in the comments or drop me an email and I’ll explain it and add it to this post.
This is actually really great, you write in a way that actually makes science fun to read and understand. Please do more IGCSE sciences!
Thanks! I’ll try do more but the syllabus has changed since I did GCSE so it takes a bit longer haha