What is Metabolism?
Metabolic reactions take place in highly controlled aqueous environments.
Metabolism is the general name given to chemical reactions that occur inside the body in order for organisms to grow, reproduce and for homeostasis to occur. These reactions are generally catalysed by enzymes.
These reactions take place in the cytoplasms of cells which is roughly 90% water (aqueous). The conditions in the cytoplasm is strictly controlled by homeostasis.
Things that are controlled:
– pH (by buffers).
– Concentrations of substances (by active transport and diffusion).
– Temperature (sweat etc).
ANABOLIC AND CATABOLIC REACTIONS
Reactions of breakdown are called catabolism and reactions of synthesis are called anabolism.
Examples:
– Respiration (glucose to H2O + CO2).
– Breakdown of Proteins into amino acids.
– Breakdown of Lipids into fatty acids.
– Breakdown of starch into glucose (digestion in humans).
Anabolic reactions are the opposite of catabolic reactions. They use energy to synthesise larger molecules from smaller ones.
Examples:
– Photosynthesis (CO2 + H2O into glucose).
– Synthesis of proteins from amino acids.
– Synthesis of glucose into starch and cellulose (growth and energy storage in plants).
PHOTOSYNTHESIS AND (AEROBIC) RESPIRATION
This is chemistry and not biology, so we only need to know the basics of this, which is some basic biology, and the formula.
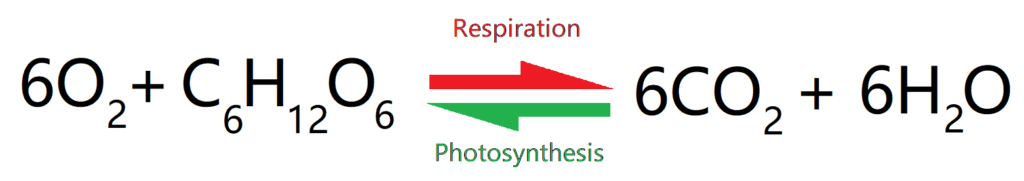
The reactions for photosynthesis and aerobic respiration are opposites of each other. However, they don’t occur via the same reactions. Both are complex and have a huge number of steps and intermediates.
The use of summary equations of photosynthesis and respiration to explain the potential balancing of oxygen and carbon dioxide in the atmosphere.
ANAEROBIC RESPIRATION
ANIMALS
Anaerobic respiration is respiration, but without oxygen. This is what happens in animals when they don’t have enough oxygen to create the amount of energy needed in a short period of time during physical activity. In this case, glucose is broken down into lactic acid to release energy.
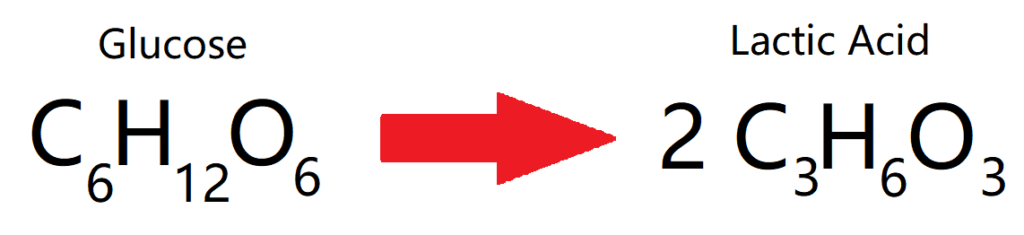
Once we return to normal aerobic respiration, our body metabolises (gets rid of) this acid.
PLANTS
In plants and microorganisms however, anaerobic respiration produces ethanol and carbon dioxide instead of lactic acid. Sound familiar? This is how many alcoholic drinks such as beer are brewed from yeast.

CONDENSATION AND HYDROLYSIS REACTIONS
Explanation of the difference between condensation and hydrolysis reactions.
Condensation reactions are reactions where two molecules combine together to form a larger molecule (common in organic chem) with the elimination of a small molecule which is usually water.
Examples:
– Amino acids forming proteins.
– Glucose forming starch (monosaccharides forming polysaccharides).
Hydrolysis reactions on the other hand, are the reverse of condensation reactions. A molecule is hydrolysed when a water molecule (often in the presence of acid or base) breaks a bond in a larger molecule to form 2 smaller molecules.
Examples:
– Proteins forming amino acids.
– Starch forming glucose (polysaccharides forming monosaccharides).