20.3 - HL Isomerism
I just need to finish optical isomers, please bear with me!
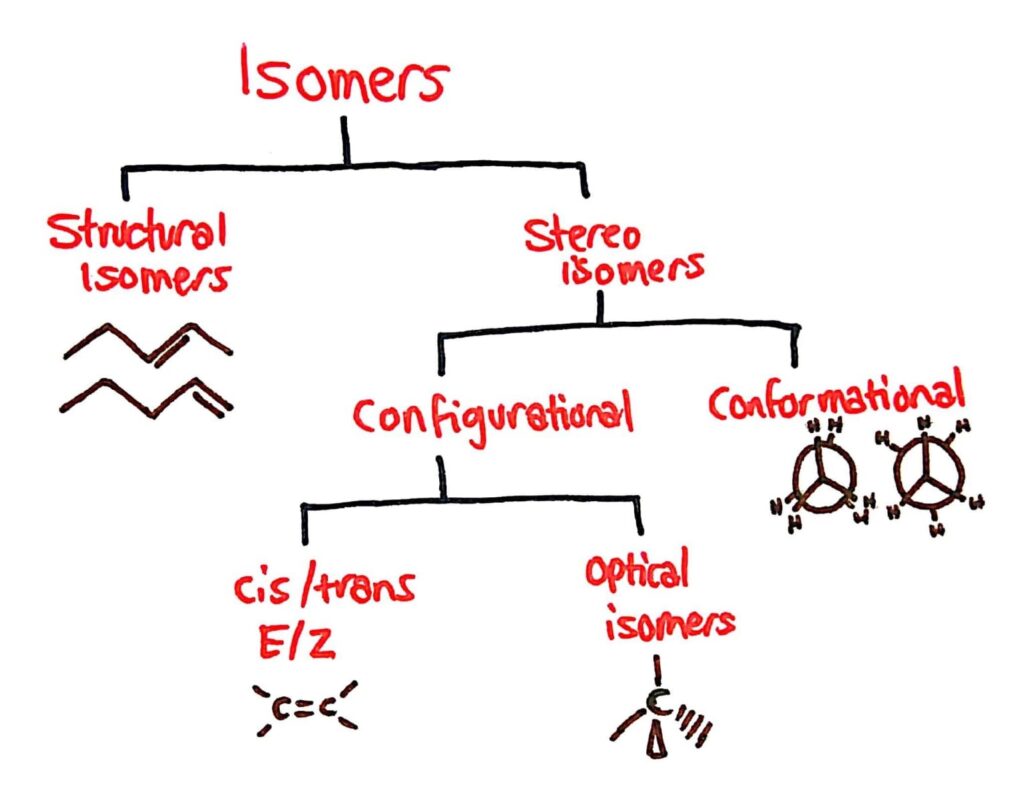
Types of Isomers
The main types of isomers you need to know are conformational, optical and cis/trans E/Z isomers.
Isomers across a double bond
Unlike single bonds, double bonds can’t rotate because of the way the pi bonds are formed between the two carbons. This means that there can be different, non-superimposable versions of the same molecule.
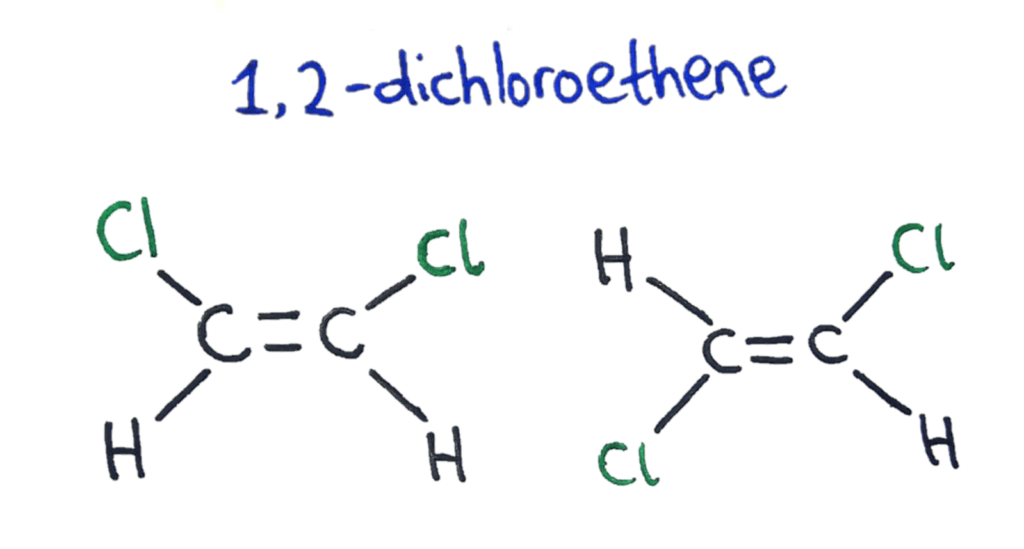
To solve the problem of naming these two configurations of functional groups around a double bond, two naming systems were developed; cis/trans and E/Z.
Cis/Trans Isomers
Cis-trans isomers can occur in alkenes or cycloalkanes (or heteroanalogues) and differ in the positions of atoms (or groups) relative to a reference plane. According to IUPAC, E/Z isomers refer to alkenes of the form R1R2 C=C R3R4 (R1≠ R2 and R3 ≠ R4) where neither R1 nor R2 need to be different from R3 or R4
The Cis/Trans naming of an isomer tells you the configuration of the molecule if it has only 2 different functional groups attached.
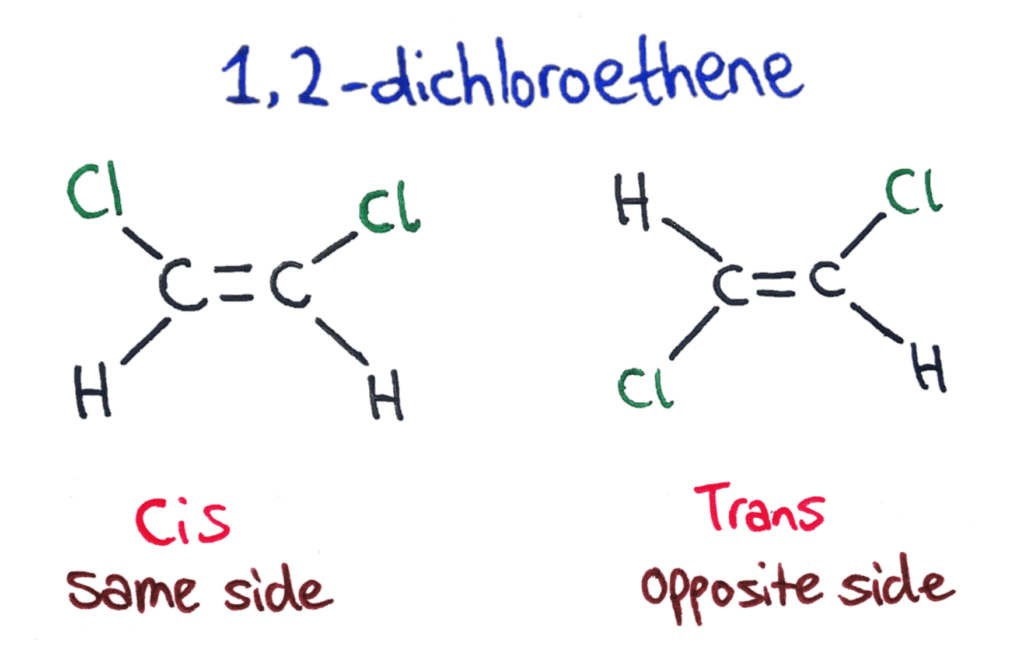
If the two groups are on the same side of the double bond then it is a cis isomer. If the two groups are on opposite sides of the double bond then is is a trans isomer.
E/Z Isomers
According to IUPAC, E/Z isomers refer to alkenes of the form R1R2 C=C R3R4 (R1≠ R2 and R3 ≠ R4) where neither R1 nor R2 need to be different from R3 or R4
The E/Z naming system was developed for molecules with more than 2 different functional groups across a double bond.
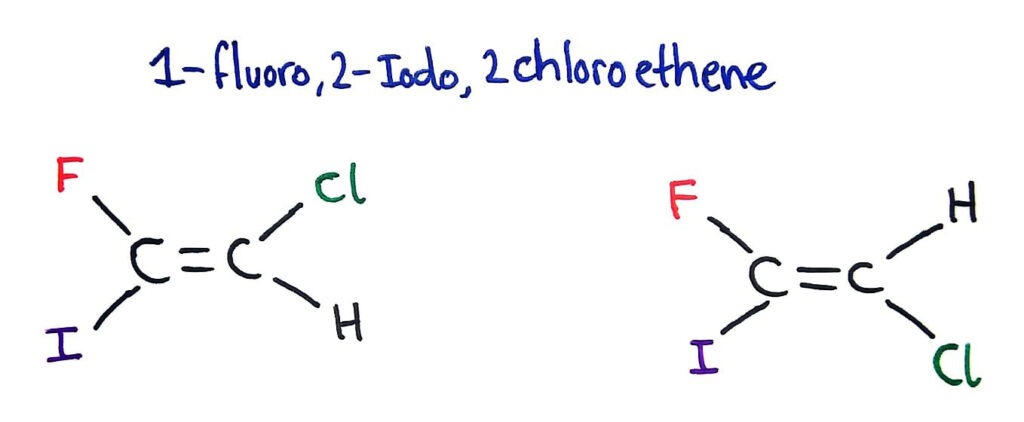
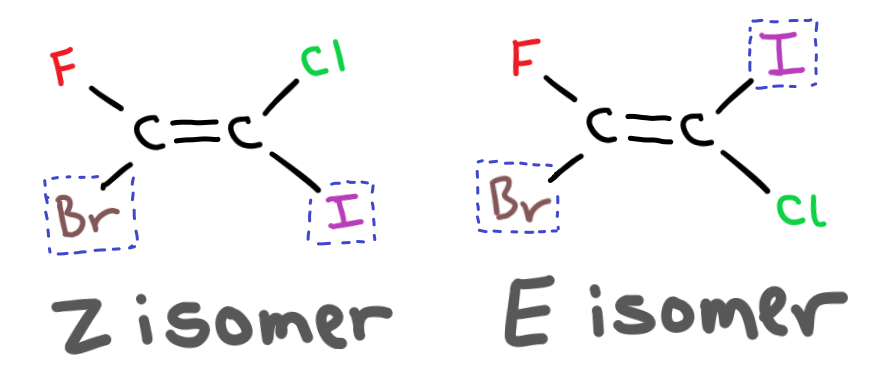
Whether the isomer is cis or trans depends on the atomic mass of the group attached. If the two heaviest groups are on the same side of the double bond then the isomer is a Z isomer.
If the two heaviest groups are on opposite sides of the double bond then the isomer is an E isomer.
Tackling more complicated molecules
It’s likely that you will come across some more difficult alkenes to classify as E or Z isomers where there are more complicated groups bonded to the double bond.
This group is usually an organic functional group where the carbon in the alkene double bond is bonded to another carbon, leading to some confusion.
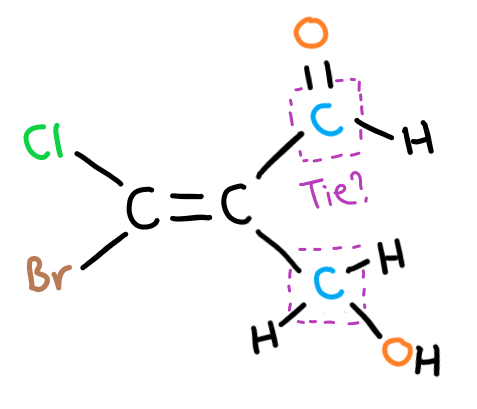
In this case, you have to look at what each of the bonds of this ‘tied’ carbon bond to. Note here to look at individual bonds of the carbon – so for the top group there are two bonds leading to an oxygen.
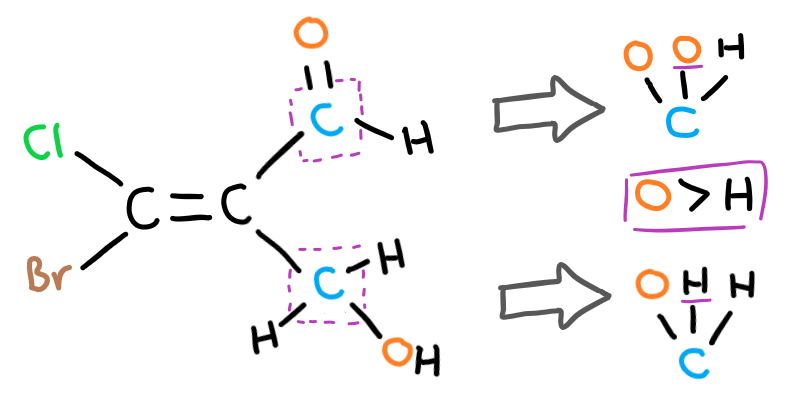
Write out the atoms that each carbon bond leads to and find the first difference between the two. In this case, the second carbon bond of the top group bonds to an oxygen while the second carbon bond in the bottom group bonds to a hydrogen. Therefore, the higher priority group is the top one and this is an E isomer.
Conformational Isomers
Stereoisomers are sub-divided into two classes – conformational isomers which interconvert by rotation about a bond and configurational isomers that interconvert only by breaking and reforming a bond.
Conformational isomers, are isomers that exist by rotation around a bond and they’re kind of weird tbh. They’re kind of a lame excuse or an isomer
So you know how a single bond can rotate around? Well that technically creates two different isomers; one where the groups on either side are aligned, and one where the groups are not aligned.
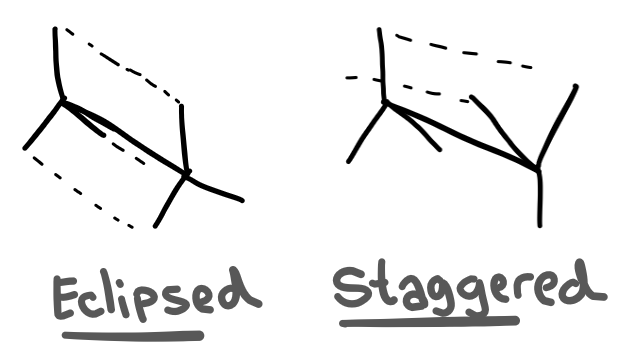
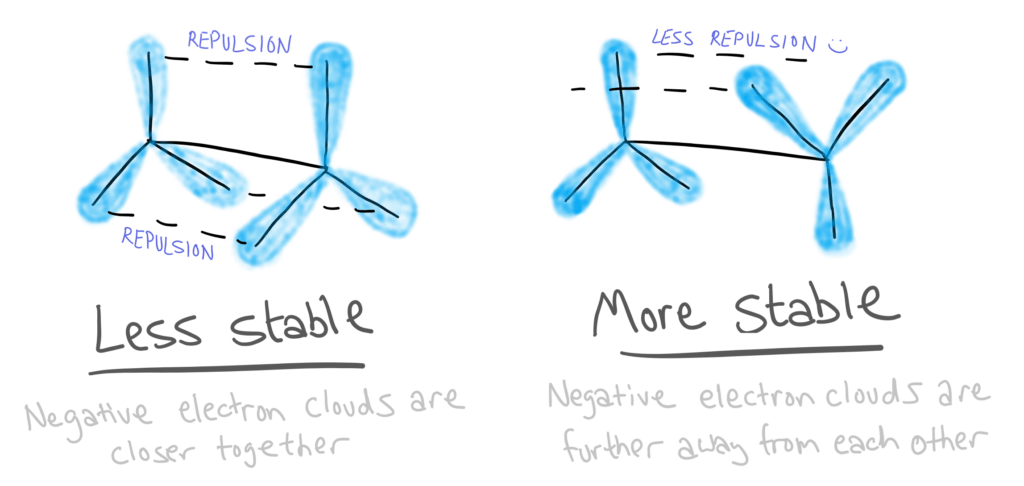
These two isomers have different stabilities due to the repulsion between electrons.
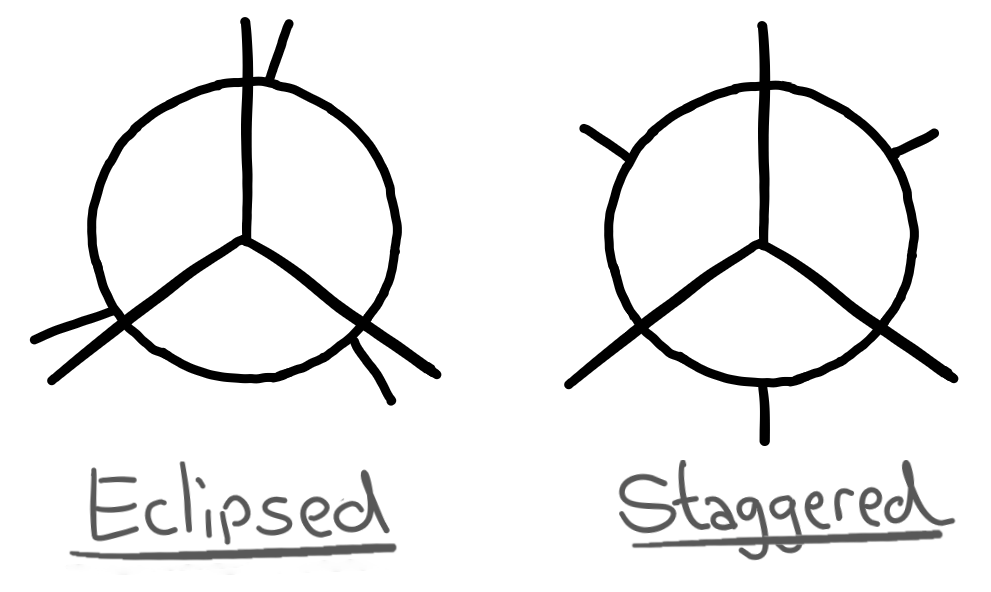
There are diagrams that represent the eclipsed and staggered configurations of the molecule which are much easier to draw.
Optical Isomers
A chiral carbon is a carbon joined to four different atoms or groups.
When you have a carbon atom bonded to 4 different functional groups, there are 2 different possible arrangements for these groups. The two different arrangements are optical isomers of each other (i’ll explain the name later) and are non-superimposable (not the same).
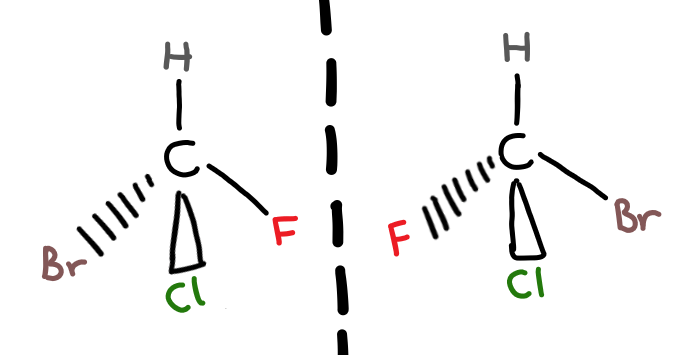
The best way to understand the difference between the two optical isomers, called enantiomers, is to physically make a model of the two.